Acid base or salt worksheet – Embark on a journey into the fascinating world of acids, bases, and salts with our captivating acid-base or salt worksheet. This comprehensive resource unravels the mysteries of these fundamental chemical concepts, providing a solid foundation for understanding their properties, reactions, and applications.
From the intricacies of the pH scale to the formation of salts, this worksheet explores the essential aspects of acid-base chemistry. Dive into real-life examples, unravel the secrets of acid-base reactions, and discover the diverse applications of these substances in various fields.
Acid-Base Properties
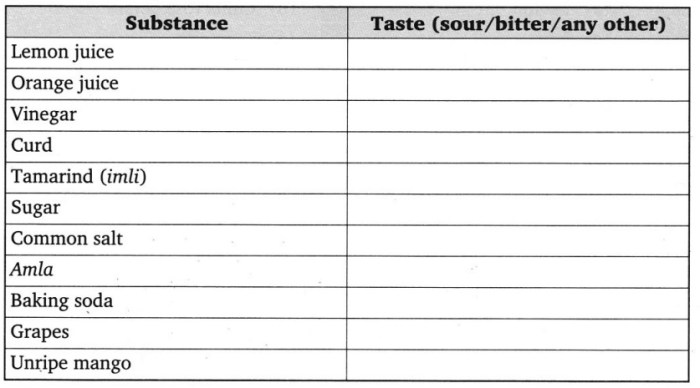
Acids, bases, and salts are chemical substances with distinct properties that play crucial roles in various chemical reactions. Understanding their behavior is essential in chemistry.
Acids are substances that release hydrogen ions (H+) when dissolved in water. They are typically sour, corrosive, and react with metals to produce hydrogen gas. Examples include hydrochloric acid (HCl), sulfuric acid (H2SO4), and acetic acid (CH3COOH).
Bases, on the other hand, are substances that release hydroxide ions (OH-) when dissolved in water. They are usually bitter, slippery to the touch, and react with acids to form salts and water. Examples include sodium hydroxide (NaOH), potassium hydroxide (KOH), and ammonia (NH3).
Salts are ionic compounds formed by the reaction of an acid with a base. They are typically neutral, meaning they do not release H+ or OH- ions in water. Examples include sodium chloride (NaCl), potassium nitrate (KNO3), and calcium carbonate (CaCO3).
The pH Scale
The pH scale is a measure of the acidity or alkalinity of a solution. It ranges from 0 to 14, with 0 being the most acidic and 14 being the most alkaline. A pH of 7 is neutral. Acids have a pH below 7, while bases have a pH above 7.
The pH scale is logarithmic, meaning that a change of one pH unit represents a tenfold change in the concentration of H+ ions. For example, a solution with a pH of 3 has ten times more H+ ions than a solution with a pH of 4.
Acid-Base Reactions
Acid-base reactions are chemical reactions that involve the transfer of protons (H+ ions) between reactants. These reactions are crucial in many chemical and biological processes.
While working on the acid base or salt worksheet, I stumbled upon a citation question. I quickly looked it up and found an excellent resource on a doll’s house citation mla . It helped me understand the proper format for citing a play.
With that knowledge, I returned to my acid base or salt worksheet and confidently completed the assignment.
Types of Acid-Base Reactions
There are three main types of acid-base reactions:
- Neutralization reactions: These reactions occur between an acid and a base, resulting in the formation of a salt and water. For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) produces sodium chloride (NaCl) and water (H2O):
HCl + NaOH → NaCl + H2O
- Acid-metal reactions: These reactions occur between an acid and a metal, resulting in the formation of a salt and hydrogen gas (H2). For example, the reaction between hydrochloric acid (HCl) and zinc (Zn) produces zinc chloride (ZnCl2) and hydrogen gas (H2):
HCl + Zn → ZnCl2 + H2
- Acid-carbonate reactions: These reactions occur between an acid and a carbonate, resulting in the formation of a salt, carbon dioxide gas (CO2), and water (H2O). For example, the reaction between hydrochloric acid (HCl) and sodium carbonate (Na2CO3) produces sodium chloride (NaCl), carbon dioxide gas (CO2), and water (H2O):
HCl + Na2CO3 → 2NaCl + CO2 + H2O
Neutralization
Neutralization is a special type of acid-base reaction that occurs when an acid and a base react in stoichiometric proportions, resulting in the formation of a salt and water. The resulting solution is neutral, meaning it has a pH of 7.
Neutralization reactions are important in many applications, such as:
- Acid spills: Neutralizing acids with bases can prevent damage to materials and skin.
- Antacids: Antacids neutralize stomach acid to relieve heartburn and indigestion.
- Water treatment: Neutralization is used to remove acidity or alkalinity from water supplies.
Everyday Examples of Acid-Base Reactions
Acid-base reactions occur in many everyday situations, including:
- Baking: Baking soda (sodium bicarbonate) is a base that reacts with acidic ingredients like lemon juice or buttermilk to produce carbon dioxide gas, which causes baked goods to rise.
- Digestion: Stomach acid (hydrochloric acid) helps break down food, and antacids neutralize stomach acid to relieve discomfort.
- Cleaning: Acids and bases are used in cleaning products to remove dirt and grime.
Salt Formation: Acid Base Or Salt Worksheet
When an acid and a base react, they form a salt and water. This process is known as neutralization. Salts are ionic compounds that contain positively charged ions (cations) and negatively charged ions (anions). The cations come from the base, and the anions come from the acid.
Salts are typically crystalline solids that are soluble in water. They have a variety of properties, including:
- They are generally neutral in pH.
- They are good conductors of electricity when dissolved in water.
- They have high melting and boiling points.
Examples of Salt Formation Reactions
Here are some examples of salt formation reactions:
- Hydrochloric acid (HCl) + sodium hydroxide (NaOH) → sodium chloride (NaCl) + water (H 2O)
- Sulfuric acid (H 2SO 4) + calcium hydroxide (Ca(OH) 2) → calcium sulfate (CaSO 4) + water (H 2O)
- Nitric acid (HNO 3) + potassium hydroxide (KOH) → potassium nitrate (KNO 3) + water (H 2O)
Acid-Base Titration
Acid-base titration is a technique used to determine the concentration of an unknown acid or base by reacting it with a solution of known concentration, called the titrant. The equivalence point is reached when the moles of acid are equal to the moles of base.
Indicators are substances that change color at or near the equivalence point, signaling the completion of the reaction.
Procedure of Acid-Base Titration
- Measure an accurately known volume of the unknown acid or base into a flask.
- Add a few drops of indicator to the flask.
- Slowly add the titrant from a burette to the flask while swirling constantly.
- Observe the color change of the indicator, indicating the equivalence point.
- Record the volume of titrant used to reach the equivalence point.
Applications of Acid-Base Titration, Acid base or salt worksheet
- Determining the concentration of acids and bases in various solutions.
- Analyzing the purity of food and drug products.
- Monitoring industrial processes involving acids and bases.
- Determining the equivalence point in acid-base reactions for stoichiometric calculations.
Applications of Acids, Bases, and Salts
Acids, bases, and salts play vital roles in various fields, ranging from industries and agriculture to everyday life. Their properties and reactivity make them indispensable in maintaining pH balance and facilitating numerous processes.
In Industries
- Acid Batteries:Lead-acid batteries are commonly used in automobiles, providing electrical energy through electrochemical reactions involving sulfuric acid.
- Fertilizer Production:Acids like nitric acid and sulfuric acid are used to produce fertilizers such as ammonium nitrate and superphosphate, essential for crop growth.
- Petroleum Refining:Acids are used as catalysts in petroleum refining processes, aiding in the conversion of crude oil into gasoline, diesel, and other products.
In Agriculture
- Soil Amendment:Agricultural lime (calcium carbonate) is added to acidic soils to neutralize acidity and improve soil pH for optimal plant growth.
- Pesticide Formulation:Acids and bases are used in the production of pesticides, which help protect crops from pests and diseases.
- Fermentation:Acids, such as lactic acid, are used in the fermentation of dairy products like cheese and yogurt, enhancing their flavor and preservation.
In Everyday Life
- Household Cleaning:Acids like hydrochloric acid and bases like sodium hydroxide are used in household cleaners to remove dirt, stains, and bacteria.
- Food Preservation:Acids like vinegar and citric acid are used as preservatives in food products to inhibit microbial growth and extend shelf life.
- pH Regulation:Bases like baking soda and acids like lemon juice are used to regulate pH levels in swimming pools, hot tubs, and other water sources.
In Biological Systems
Acids, bases, and salts play a crucial role in maintaining pH balance in biological systems. The pH of bodily fluids, such as blood, is tightly regulated to ensure optimal enzyme activity and cellular function. Deviations from the normal pH range can lead to severe health consequences.
Safety Considerations
Handling acids, bases, and salts requires utmost caution due to their potentially hazardous nature. Understanding the proper safety measures and disposal methods is crucial to prevent accidents and protect both individuals and the environment.
Acids and bases are corrosive substances that can cause severe burns to the skin, eyes, and respiratory system. Salts, although generally less reactive, can still pose risks such as irritation or toxicity if ingested or inhaled.
Safety Precautions
- Wear appropriate personal protective equipment (PPE): This includes gloves, safety glasses, and a lab coat to prevent direct contact with the substances.
- Handle chemicals in a well-ventilated area: Fumes or vapors released from acids and bases can be harmful if inhaled.
- Never mix acids and bases directly: This can result in a violent reaction, releasing heat and potentially hazardous gases.
- Always add acid to water, not vice versa: Adding water to acid can cause a splashing reaction due to the release of heat.
- Use caution when diluting concentrated acids: Slowly add acid to water while stirring constantly to avoid excessive heat generation.
- Store chemicals properly: Acids and bases should be stored in separate, labeled containers in a cool, dry place.
Disposal Methods
Proper disposal of acids, bases, and salts is essential to minimize environmental impact and prevent potential hazards.
- Neutralize acids and bases before disposal: This can be done by carefully mixing them with a neutralizing agent such as sodium bicarbonate or sodium hydroxide.
- Dispose of neutralized solutions according to local regulations: Contact your local waste management authority for specific guidelines.
- Dispose of salts as non-hazardous waste: However, some salts may require special disposal methods if they contain hazardous components.
Potential Hazards and Safety Measures
| Hazard | Safety Measure |
|---|---|
| Skin contact with acids/bases | Wear gloves and rinse affected area immediately with plenty of water |
| Eye contact with acids/bases | Wear safety glasses and flush eyes with water for at least 15 minutes |
| Inhalation of acid/base fumes | Work in a well-ventilated area and wear a respirator if necessary |
| Ingestion of acids/bases | Do not induce vomiting; seek medical attention immediately |
| Skin contact with salts | Wash affected area with water |
| Inhalation of salt dust | Wear a dust mask |
| Ingestion of salts | Follow instructions on the product label or seek medical advice |
Detailed FAQs
What is the pH scale?
The pH scale is a measure of the acidity or alkalinity of a solution, ranging from 0 to 14. A pH of 7 indicates neutrality, while values below 7 indicate acidity and values above 7 indicate alkalinity.
What is the difference between an acid and a base?
Acids release hydrogen ions (H+) in water, while bases release hydroxide ions (OH-). Acids have a sour taste and can react with metals to produce hydrogen gas. Bases have a bitter taste and feel slippery to the touch.
What is salt formation?
Salt formation occurs when an acid reacts with a base, neutralizing each other and forming a salt and water. Salts are ionic compounds that typically have a neutral pH.