Select the true statement about dehydration synthesis or hydrolysis. – As the title suggests, this discourse delves into the realm of dehydration synthesis and hydrolysis, unveiling their intricate processes, contrasting their distinct characteristics, and exploring their multifaceted applications. Brace yourself for an enlightening journey that will illuminate the depths of these fundamental chemical reactions.
In the realm of biochemistry, dehydration synthesis and hydrolysis stand as two pivotal processes that govern the intricate dance of molecular transformations. Dehydration synthesis, the master of bond formation, orchestrates the union of two molecules, expelling a water molecule in its wake.
Hydrolysis, on the other hand, assumes the role of a molecular dismantler, cleaving bonds with the assistance of water molecules.
Dehydration Synthesis
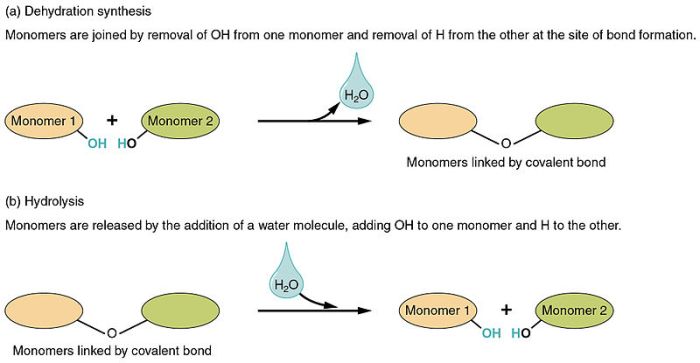
Dehydration synthesis is a chemical reaction that involves the removal of a water molecule from two molecules to form a new, larger molecule. The process is typically catalyzed by an enzyme and requires energy input. Dehydration synthesis reactions are common in biological systems, such as the formation of proteins and carbohydrates.An
example of a dehydration synthesis reaction is the formation of a peptide bond between two amino acids. In this reaction, the amino group of one amino acid reacts with the carboxyl group of another amino acid, releasing a water molecule and forming a peptide bond.
Hydrolysis
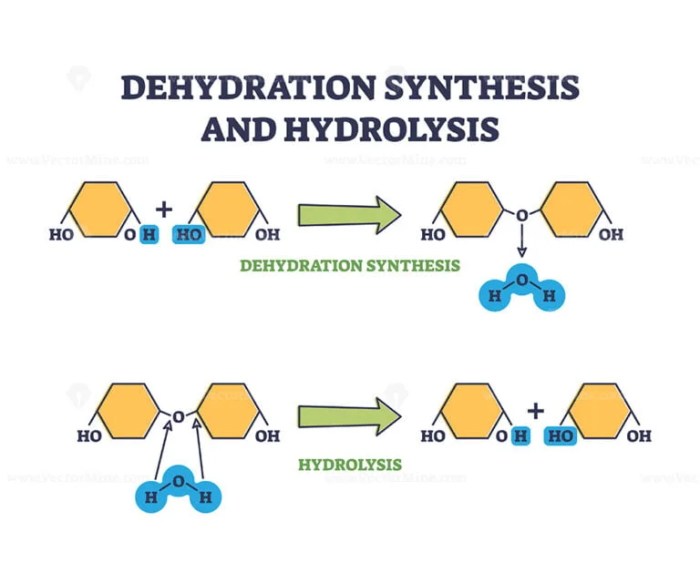
Hydrolysis is a chemical reaction that involves the addition of a water molecule to a molecule, breaking it down into smaller molecules. Hydrolysis reactions are typically catalyzed by an enzyme and require energy input. Hydrolysis reactions are common in biological systems, such as the digestion of food and the breakdown of waste products.An
example of a hydrolysis reaction is the digestion of starch into glucose. In this reaction, the starch molecule is broken down into smaller glucose molecules by the enzyme amylase.
Essential FAQs: Select The True Statement About Dehydration Synthesis Or Hydrolysis.
What is the primary distinction between dehydration synthesis and hydrolysis?
Dehydration synthesis joins molecules by removing water, while hydrolysis breaks them apart by adding water.
Can you provide an example of a dehydration synthesis reaction?
The formation of a peptide bond between two amino acids is a classic example of dehydration synthesis.
What is the significance of enzymes in these processes?
Enzymes act as catalysts, dramatically increasing the rate of dehydration synthesis and hydrolysis reactions.
