Gizmos student exploration ionic bonds embarks on an enlightening journey, delving into the fundamental concepts of ionic bond formation, properties, and applications. This exploration provides a comprehensive understanding of ionic compounds, equipping learners with a deeper appreciation for their significance in the world around us.
Through interactive simulations and engaging activities, gizmos student exploration ionic bonds fosters a dynamic learning environment that empowers students to actively engage with the subject matter. This immersive approach fosters a deeper understanding of the underlying principles governing ionic bond formation, enhancing their ability to apply this knowledge to real-world scenarios.
1. Gizmos Student Exploration
Ionic Bonds
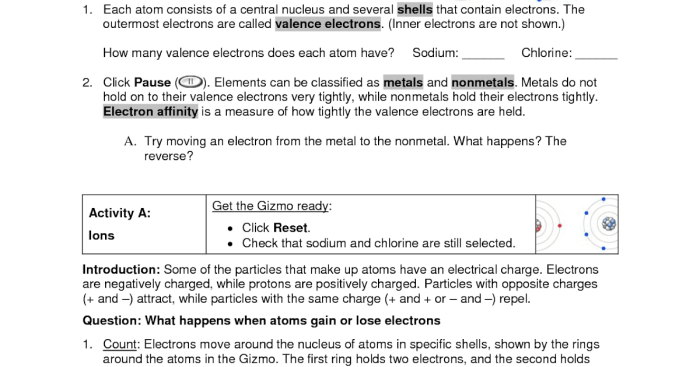
Ionic bonds are a type of chemical bond formed between atoms of metals and non-metals. In the gizmos student exploration, students can investigate the formation of ionic bonds between various elements.
Examples of Ionic Bonds
- Sodium (Na) and chlorine (Cl) form NaCl (table salt)
- Potassium (K) and iodine (I) form KI (potassium iodide)
- Calcium (Ca) and fluorine (F) form CaF 2(fluorite)
Enhancing Understanding with Gizmos
Gizmos student exploration provides interactive simulations and visualizations that allow students to:
- Observe the transfer of electrons between atoms
- Predict the charge of ions formed
- Explore the relationship between atomic number and ion formation
2. Properties of Ionic Compounds
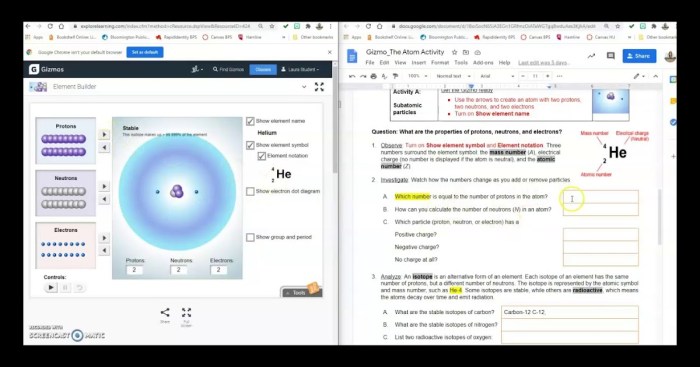
Physical Properties
- High melting and boiling points
- Solid at room temperature
- Brittle and crystalline
Chemical Properties, Gizmos student exploration ionic bonds
- React with water to form electrolytes
- Form ionic crystals
- High solubility in polar solvents
Ionic Bond Strength and Properties
The strength of an ionic bond is influenced by:
- Charge of the ions
- Size of the ions
- Lattice energy
- Table salt (NaCl): Seasoning, food preservation
- Potassium iodide (KI): Thyroid health
- Calcium fluoride (CaF 2): Dental fluoridation, optics
- Sodium chloride (NaCl): Deicing agent
- Magnesium oxide (MgO): Antacid
- Food industry: Preservatives, flavor enhancers
- Medical industry: Pharmaceuticals, diagnostic agents
- Chemical industry: Catalysts, reactants
- Electronics industry: Semiconductors, insulators
- Excess sodium intake: Hypertension, cardiovascular disease
- Fluoride in drinking water: Dental fluorosis
- Chloride pollution in water: Aquatic toxicity
Stronger ionic bonds lead to higher melting and boiling points, greater hardness, and lower solubility.
| Property | Description |
|---|---|
| Melting point | High due to strong electrostatic forces |
| Boiling point | High due to strong electrostatic forces |
| Solubility | High in polar solvents due to ion-dipole interactions |
3. Applications of Ionic Compounds: Gizmos Student Exploration Ionic Bonds
Real-World Examples
Importance in Industries
Environmental and Health Implications
Some ionic compounds can have environmental and health implications:
FAQ Insights
What are the key features of gizmos student exploration ionic bonds?
Gizmos student exploration ionic bonds offers a range of interactive simulations, engaging activities, and assessment tools that facilitate a comprehensive exploration of ionic bond formation, properties, and applications.
How can gizmos student exploration ionic bonds enhance student learning?
By providing a hands-on, interactive learning experience, gizmos student exploration ionic bonds helps students visualize and understand the abstract concepts of ionic bonding. The simulations allow students to manipulate variables and observe the resulting changes, fostering a deeper understanding of the underlying principles.
What are the benefits of using gizmos student exploration ionic bonds in the classroom?
Gizmos student exploration ionic bonds provides educators with a valuable resource to engage students and make learning more interactive and enjoyable. The simulations and activities align with curriculum standards and can be easily integrated into lesson plans, enhancing student engagement and promoting deeper understanding.